Metals are well known for their ability to conduct electricity and heat, but have you ever stopped to think why they conduct electricity? In this article we will explore the science behind why metals are such a very good conductors of electricity and how conductivity works?
In simple words, metals conduct electricity because they have many free electrons in their outermost energy levels/orbit. These electrons are not bound to any atom and are able to move freely throughout the metal structure.
But the question is why do metals have got many free electrons in the first place? It all comes from the atomic structure of the metals. Metals have atoms that are arranged in a regular, repeating pattern known as a crystal lattice structure. Within this lattice structure, the atoms are held together by an array of bonds between their outermost energy levels, or valence electrons. The bond and the lattice structure are important for the integrity of the metals but not just the metals every element has its lattice structure.
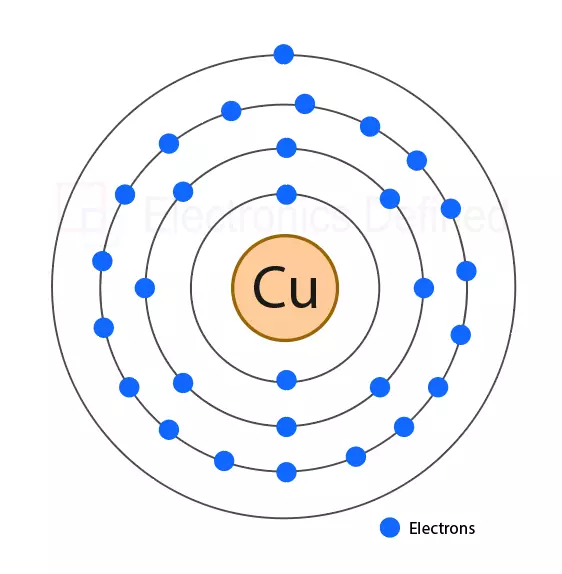
Atoms that have 4 or less valance electrons are mostly conductive, atom with single valance electron are best conductors of electricity like copper. Elements with 4 electrons on outer shell are mostly semiconductors. Nonmetals won’t have any free electrons and their outer energy level will be always filled with electrons thus, they do not conduct electricity.
Figure below shows an atom of the copper metal where the outer orbit/ shell has only one electron in outer energy level making copper a good conductor.
In any element the atoms can hold maximum electron is calculated by 2(n^2) [2n squared] where n is the shell number. Calculation for 2nd cell will be:
2(2^2)
2×4
=8 electrons
However, in case of metals the outermost energy level or orbit is not filled with the electrons due to the lattice structure of the metals. Due to same lattice structure the metals have free electron that can move around the lattice, these free electrons are responsible for the ability of metals to conduct electricity.
In insulators the outermost energy level is filled thus they do not provide any free electrons and they are basically insulators.
Image below shows atomic structure of neon that is a very good insulator of electricity. If you notice the outer shell/ orbit is filled with electrons that makes neon to be insulator and it does not conduct electricity.

When an electric current is applied to a metal, the free electrons can move easily throughout the lattice, creating a flow of electrons that is basically known as current. Therefore, metals are such good conductors of electricity.
The way electrons flow through the metal is, new electrons come and push existing electrons creating a situation of excess electrons. However, the metal should release an electron if one electron is gained to stay stable, so the electron moves from metal and goes to low voltage side. This cycle keeps repeating until the source of electricity is removed. Metals do not keep or remove any new electrons during flow of electricity they just move incoming or existing electrons.
The best electricity conductor is silver unfortunately its very expensive and not easily available. Nevertheless, silver and gold are used in electronics where optimal conductivity is required for example silver is used to connect solar cells of satellites even CPUs and many chips contain gold.
Copper is the second-best conductor of electricity and its quite cheaper than silver, that ultimately makes the copper the best conductor for electronics. Other metals with high conductivity include aluminium, Lithium, zinc etc.

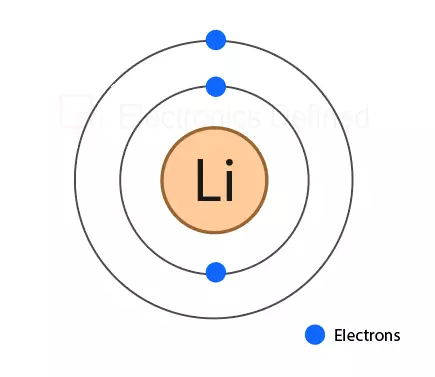
Figure below show the atom of lithium metal that is most widely used for making li-ion and li-polymer batteries. Lithium provides one free electron, making it a good conductor.
Ability to conduct electricity is just one of the useful properties of metals. Despite their electrical conductivity, metals are also heat conductive. In addition, metals are also strong, durable, and malleable, ductile which makes them ideal for a wide range of applications.
For example, metals are used in the construction of buildings and infrastructure, as well as in the manufacturing of cars, airplanes, and other forms of transportation. They are also used in the production of electrical wires and cables, essential for transmitting electricity and electrical signals.
List metals according to their current flow ability
Here is a quite a big list of metals in ordered according to electrical conductivity, or their ability to allow the flow of electric current through them.
Metals conductivity list:
- Silver: Silver is the most electrically conductive metal, with a conductivity of about 63 x 10^6 S/m (Siemens per meter).
- Copper: Copper is a very good conductor of electricity, with a conductivity of about 59 x 10^6 S/m.
- Gold: Gold has a conductivity of about 46 x 10^6 S/m, making it a good conductor of electricity.
- Aluminum: Aluminum has a conductivity of about 37 x 10^6 S/m, making it somewhat a good conductor of electricity.
- Nickel: Nickel has a conductivity of about 21 x 10^6 S/m, making it a somewhat poor conductor of electricity.
- Iron: Iron has a conductivity of about 9 x 10^6 S/m, making it a poor conductor of electricity.
- Lead: Lead has a conductivity of about 6 x 10^6 S/m, making it a very poor conductor of electricity.
- Tin: Tin has a conductivity of about 5.8 x 10^6 S/m, making it a very poor conductor of electricity.
- Zinc: Zinc has a conductivity of about 5.9 x 10^6 S/m, making it a very poor conductor of electricity.
- Steel: Steel has a conductivity of about 6 x 10^6 S/m, making it a very poor conductor of electricity.
- Platinum: Platinum has a conductivity of about 10 x 10^6 S/m, making it a poor conductor of electricity.
- Palladium: Palladium has a conductivity of about 9.2 x 10^6 S/m, making it a poor conductor of electricity.
- Mercury: Mercury has a conductivity of about 9.1 x 10^6 S/m, making it a poor conductor of electricity.
- Cobalt: Cobalt has a conductivity of about 6.7 x 10^6 S/m, making it a very poor conductor of electricity.
- Manganese: Manganese has a conductivity of about 6.4 x 10^6 S/m, making it a very poor conductor of electricity.
- Chromium: Chromium has a conductivity of about 6.3 x 10^6 S/m, making it a very poor conductor of electricity (very expensive metal even expensive than silver).
- Molybdenum: Molybdenum has a conductivity of about 5.9 x 10^6 S/m, making it a very poor conductor of electricity.
- Tungsten: Tungsten has a conductivity of about 5.5 x 10^6 S/m, making it a very poor conductor of electricity.
- Titanium: Titanium has a conductivity of about 5.2 x 10^6 S/m, making it a very poor conductor of electricity.
- Nickel-Iron Alloys: Nickel-Iron alloys, such as Invar and Kovar, have conductivities of about 5 x 10^6 S/m, making them very poor conductors of electricity.
Note: Values may be different because different sources provide different results.
Resistivity list of metals
Metals are very good conductors or electricity, but they are not perfect at all in fact they also have some amount resistance to flow of current. This is basically called resistivity of metals. The resistivity of a metal against the flow of electricity is usually measured in units of ohm-meters (Ω⋅m). The resistivity of a metal may vary depending on factors such as its thickness, length, temperature, composition, and the presence of impurities in metal. Some of the most resistive metals include zinc, iron, nichrome etc.
List below represents metals resistivity and conductivity in ascending:
| Material | ρ (Ω•m) at 20 °C Resistivity | σ (S/m) at 20 °C Conductivity |
| Silver | 1.59×10−8 | 6.30×107 |
| Copper | 1.68×10−8 | 5.96×107 |
| Annealed copper | 1.72×10−8 | 5.80×107 |
| Gold | 2.44×10−8 | 4.10×107 |
| Aluminum | 2.82×10−8 | 3.5×107 |
| Calcium | 3.36×10−8 | 2.98×107 |
| Tungsten | 5.60×10−8 | 1.79×107 |
| Zinc | 5.90×10−8 | 1.69×107 |
| Nickel | 6.99×10−8 | 1.43×107 |
| Lithium | 9.28×10−8 | 1.08×107 |
| Iron | 1.0×10−7 | 1.00×107 |
| Platinum | 1.06×10−7 | 9.43×106 |
| Tin | 1.09×10−7 | 9.17×106 |
| Carbon steel | (1010) | 1.43×10−7 |
| Lead | 2.2×10−7 | 4.55×106 |
| Titanium | 4.20×10−7 | 2.38×106 |
| Grain oriented electrical steel | 4.60×10−7 | 2.17×106 |
| Manganin | 4.82×10−7 | 2.07×106 |
| Constantan | 4.9×10−7 | 2.04×106 |
| Stainless steel | 6.9×10−7 | 1.45×106 |
| Mercury | 9.8×10−7 | 1.02×106 |
| Nichrome | 1.10×10−6 | 9.09×105 |
Note: Values may be different because different sources provide different results.
List of resistivity of non-metals:
| Carbon (amorphous) | 5×10−4 to 8×10−4 | 1.25 to 2×103 |
| Carbon (graphite) | 2.5×10−6 to 5.0×10−6 | 2 to 3×105 |
| Carbon (diamond) | 1×1012 | ~10−13 |
| Germanium(semiconductor) | 4.6×10−1 | 2.17 |
| Sea water | 2×10−1 | 4.8 |
| Drinking water | 2×101 to 2×103 | 5×10−4 to 5×10−2 |
| Silicon(semiconductor) | 6.40×102 | 1.56×10−3 |
| Wood (damp) | 1×103 to 4 | 10−4 to 10-3 |
| Deionized water | 1.8×105 | 5.5×10−6 |
| Glass | 10×1010 to 10×1014 | 10−11 to 10−15 |
| Hard rubber | 1×1013 | 10−14 |
| Wood (oven dry) | 1×1014 to 16 | 10−16 to 10-14 |
| Sulphur | 1×1015 | 10−16 |
| Air | 1.3×1016 to 3.3×1016 | 3×10−15 to 8×10−15 |
| Paraffin wax | 1×1017 | 10−18 |
| Fused quartz | 7.5×1017 | 1.3×10−18 |
| PET | 10×1020 | 10−21 |
| Teflon | 10×1022 to 10×1024 | 10−25 to 10−23 |
Note: Values may be different because different sources provide different results.
Metals resist current flow when heated
Heat can flow through a metal in a process known as thermal conductivity. Electrons are important for heat flow in metals. The way free electrons are required for current flow, same way free electrons are necessary for the flow of heat through the metals. When the metal is heated the free electrons move and displace the heat to the colder side of the metal.
However, if the metal is heated the current flow will be decreased because heat affects the flow of free electrons thus the resistivity will increase. In simple words resistivity of metals increase when they are heated.
Superconductivity is a phenomenon of metal, where if metals are cooled down at a specific critical temperature, they become superconductors. Super conductors are conductor that do not have any resistance to flow of electrical current! Yes, no resistance to flow of current at all. But the superconductivity comes at price the metal should be cooled down to a very low temperature that is called critical temperature that is mostly lower than -100 degree Celsius. Aluminium are mercury are some the well-known metals for their super conductivity.
Usage of metal totally depends on the application. Electric circuit boards are designed keeping these factors in mind to provide proper functioning of the device and long-term reliability.



